Hubei Biocause Phamaceutical Co., Ltd.
#122Yangwan Road,Jingmen, Hubei, China
Tel.:++86 (0)27 65651730 / 85628285
Fax.:++86 (0)27 65652329
Email:export@biocause.com,export@biocause.net
CONTRACT MANUFACTURE
Contract Manufacture
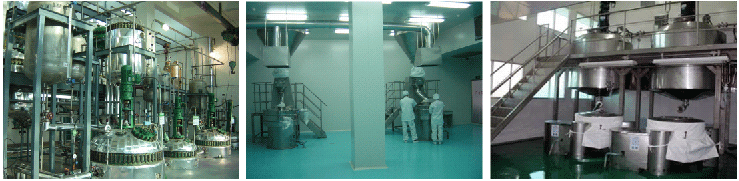
We have 3 API GMP facilities,
involves multifunctional workshops and kilo pilot plants
with total of 510 reaction vessels at max5000Land over1000 m total reaction
volumes for annual output reaching 4000 metric tons.
◆Isolation technology, including purification, controlled
crystallization and industrial
scale column chromatography
◆Operational conditions, typically ranging from-90 Cto280 C, and from low
pressure to 50 bars
◆Quick turnaround of manufacture proposals
◆Scale up of multi-step processes
◆Various purification capabilities to achieve high purities
(>99%)
◆Reduce cost through cycle time reduction and continuous
process optimization
◆Effective bulk material sourcing & extensive analytical
support
◆Daily engineering support to generate scale up parameters
◆High degree of EHS regulations compliance
Copyright © 2004 - 2013 BIOCAUSE Inc. All Rights Reserved.
